Phesi Inc., a data-driven provider of AI-powered clinical development analytics products and solutions, has analyzed protocol design data from 1,324,820 US patients, from Phase 2 and 3 single country clinical trials across a total of 1,580 cohorts. This data set represents 495 disease conditions with trials starting from 2010-2020. The results showed that Asian, Hispanic and Latino, Native Americans and Alaska natives, Native Hawaiians and other Pacific Islander patient subpopulations were all significantly and consistently underrepresented over the decade (fig.1.).
“The number of patient subgroups is increasing as we expand our understanding of how therapies affect certain demographics and continue to research genetic biomarkers,” commented Dr Gen Li, President, Phesi. “Trial sponsors are making a concerted effort to address the lack of ethnic group representation, and there is also growing pressure from the public as understanding of clinical trials is increasing following the COVID-19 pandemic. More than 70% of the population believe patients need more access to clinical trials and opportunities to participate.[1] The demand is clearly there for wider inclusion, and to meet it, sponsors must become more data-driven to ensure that new treatments are efficacious for all ethnic and racial groups, whenever applicable.”
Dr Li added, “It’s also important that when considering diversity and inclusion that we don’t look at issues in isolation. We know that a patient’s race can affect how they respond to drugs. Pharmacogenetic differences in Asians may affect their responses to important drugs such as Warfarin and Clopidogrel, increasing and decreasing drug response respectively. The FDA has also approved Bidil (isosorbide dinitrate/hydralazine), the only heart failure medicine approved specifically for African Americans. But we’ve also seen over the last two years of the pandemic how other factors not related to race can affect outcomes. Our own research has shown that obesity is a major complicating factor and the biggest predictor of COVID-19 symptom severity. The key takeaway is that sponsors optimizing the design of their clinical trials in any disease must be better informed by leveraging the variety and volume of real-world and real-time data available to them today.”
The data (fig.1.) show Black and African American patients appear to have become better represented over the past decade, making up 14.92% of the data set, compared to 13.4% of the total US population in 2019 US Census Bureau estimates. This positive change is due to concentrated efforts from industry to improve racial diversification, as well as vital work undertaken by patient advocacy groups for diseases disproportionally impacting Black/African Americans in the US. This is a broad snapshot over trials over the last decade and the increased numbers do not mean the work to ensure true representation of Black/African American participants in clinical trials is done. Moreover, other racial groups are still markedly underrepresented in trials.
“Patient subgroups in clinical trials continue to evolve as availability of real-world data allows sponsors to design more precise and targeted trials. The demand for inclusion also extends into other characteristics like sex and age,” commented Dr Paul Chew, Chief Medical Officer at Phesi. “To further advance our understanding of diversity, we will need to see greater alignment between sponsors and government and regulatory bodies, to ensure clinically meaningful classifications are standardized. Moreover, the industry must address the barriers and justified concerns of patients due to historical treatment in trials to ensure everyone is included and fairly represented in the clinical development process.”
Dr Chew continued, “The good news for sponsors is that technology and integrated data sets are available today that are driving the development of synthetic patient profiles. This predictive analytics approach helps to address inclusion issues and to accelerate trials. In addition, the advances that we’ve seen in virtualized and decentralized trial throughout COVID-19 has shown that geography should be a factor in trial participation. We should see the results of these new technology and data-driven approaches trickle through into increased diversity in trial data.”
To find out more about Phesi, visit https://www.phesi.com/.
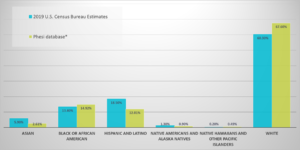
Fig. 1. Data from 1,324,820 US patients in Phase 2 and 3 single country clinical trials in 1,580 cohorts, across 495 disease conditions in trials starting from 2010-2020.

